Theme: “The voice of rare diseases - research and treatmentâ€
Rare Diseases 2016
Conference series LLC invites all the participants from all over the world to attend ‘Annual Congress on Rare Diseases and Orphan Drugs’ during Oct 26-27, 2016 in Chicago, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Rare Diseases 2016 is a global platform to discuss and learn about Rare Diseases, rare cancer, Morgellons, Renal cell carcinoma, malignant glioma, Turner syndrome, Multiple myeloma, Hepatocellular carcinoma, Diabetes in the young, Systemic Amyloidosis, Autoimmune diseases, Disorders of genetic origin, idiopathic disorders, rare genetic diseases, Orphan Drugs, Alzheimer’s Disease and many more. OMICS International organizes a conference series of 1000+ Global Events inclusive of 300+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members.
Track 1: Different types of Rare Diseases
Rare Disease is technically defined as a disease that is found in fewer than 5 people per every 10,000 people. While we’ve all heard of diseases, seen someone with a disease, and had a disease personally, it’s unlikely that we’ve encountered a rare disease. In the non-medical world, people use and interchange disease to mean infection, sickness, illness, or something similar. In the medical world, a disease is an abnormal condition that impairs bodily functions and is often associated with certain signs and symptoms. Also called an orphan disease. Most rare diseases are genetic, and thus are present throughout the person's entire life, even if symptoms do not immediately appear.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd International Conference on Chronic Obstructive Pulmonary Disease July 11-12, 2016, Australia; 2nd International Conference on Retroviruses & Novel Drugs June 30-July 01, 2016, South Africa; 3rd World Congress on Hepatitis and Liver Diseases October 17-19, 2016, UAE ;4th International Conference on Epidemiology and Public Health October 03-05, 2016, United Kingdom; 2nd International Conference on Flu November 17-19, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; Orphan Drugs & Rare Diseases; SCT 37th Annual Meeting , Canada - May 15-18, 2016; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; ERA-Net for Research Programmes on Rare Diseases March 9 - 12, 2016, Spain; Orphan Drugs & Rare Diseases Global Congress 2016 Europe March 14th – 16th 2016, UK; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016, South Africa.
Track 2: Clinical Research and Public Awareness
Clinical research is a branch of healthcare science. The first step in tackling this challenge is regularly getting the public to think about participating in clinical research. People need to consider how they can help advance the prevention, diagnosis, and treatment of disease. It is never too early to consider participation whether or not someone ultimately chooses to join a study.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd World Congress on Pharmacology August 08-10, 2016, UK ; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, Phoenix, Arizona, USA; 2ndInternationalConferenceon Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa.
Track 3: Mystery Diagnosis of Rare Diseases
Diagnostic error in medicine is common. For example, a study from an intensive care unit demonstrated nearly 20% discordance between the clinically-defined cause of death and findings at post-mortem examination. Not surprisingly; therefore, the diagnosis of rare diseases is often delayed.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 2nd International Conference on Flu November 17-19, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 3rd World Congress on Hepatitis and Liver Diseases October 17-19, 2016, UAE; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 4: Challenges in Rare Diseases Treatment
There are approximately 7,000 rare diseases, which from a regulatory perspective are defined as those diseases where there are less than 200,000 patients in the US or that affect no more than five in 10,000 of the general population in the EU. "Orphan drugs" are medicinal products intended for diagnosis, prevention or treatment of life-threatening or debilitating rare diseases. They are "orphans" because the pharmaceutical industry has little interest under normal market conditions in developing and marketing drugs intended for only a small number of patients suffering from very rare conditions.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd World Congress on Hepatitis and Liver Diseases October 17-19, 2016, UAE; 2nd International Conference on Flu November 17-19, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 5: Rare Diseases in Cancer
Rare cancers tend to be caused by simple genetic mutations, and common cancers tend to be caused by a complex set of genetic and epigenetic aberrations that continually increase in number as the tumor develops. All cancers that can be cured when in an advanced clinical stage are rare cancers. Rare cancer syndromes typically produce various types of cancers, including rare cancers and common cancers.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 14th World Congress on Cancer Therapy December 05-07, 2016 Philadelphia, Pennsylvania, USA; 3rd World Congress on Hepatitis and Liver Diseases October 17-19, 2016, UAE; 2nd International Conference on Flu November 17-19, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 6: Rare Diseases in Aging
Aging is a collection of degenerative changes that occur in organisms that lack the ability to perpetually regenerate. Age is a major risk factor for most common neurodegenerative diseases. Dementia becomes more common with age. The spectrum includes mild cognitive impairment, Alzheimer's disease, cerebrovascular disease, Parkinson's disease and Lou Gehrig's disease. Rare diseases provide much insight into the cellular processes that hasten the aging process.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, International Conference on Aging & Gerontology August 08-09, 2016 Las Vegas, Nevada, USA; 3rd World Congress on Hepatitis and Liver Diseases October 17-19, 2016, UAE; 2nd International Conference on Flu November 17-19, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 7: Infectious Diseases and Immune Deficiencies
Worldwide, about one-third of human deaths are attributable to infections. In addition, the so-called non-infectious causes of death often have a cryptic infectious etiology.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd World Congress on Hepatitis and Liver Diseases October 17-19, 2016, UAE; 2nd International Conference on Flu November 17-19, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 8: Orphan Drugs, Development trends and strategies
The growth of pharma industries has slowed in recent years because of various reasons such as patent expiries, generic competition, drying pipelines, and increasingly stringent regulatory guidelines. Many blockbuster drugs will lose their exclusivity in next 5 years. Therefore, the current economic situation plus the huge generic competition shifted the focus of pharmaceutical companies from the essential medicines to the new business model — niche busters, also called orphan drugs.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, International Conference on Medical Ethics & Legal Medicine June 09-11, 2016 , UK; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, Phoenix, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 9: Orphan Drugs and Ethical Issues
This essay outlines the moral dilemma of funding orphan drug research and development. To date, ethical aspects of priority setting for research funding have not been an issue of discussion in the bioethics debate. Conflicting moral obligations of beneficence and distributive justice appear to demand very different levels of funding for orphan drug research. The two types of orphan disease, rare diseases and tropical diseases, however, present very different ethical challenges to questions about allocation of research funds. The dilemma is analysed considering utilitarian and rights based theories of justice and moral obligations of non-abandonment and a professional obligation to advance medical science. The limitations of standard economic evaluation tools and other priority setting tools used to inform health policy decision makers on research funding decisions are outlined.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 5th Global Pharmacovigilance Summit April 28-29, 2016, UAE; 2nd Annual Congress on Pharma Middle East October 10-12, 2016; International Conference on Medical Ethics & Legal Medicine June 09-11, 2016 , UK; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; International Conference on Anatomy - Physiology Aug 11-13, 2016, UK; 2nd International Conference on Clinical Trials, August 22-24, 2016 Philadelphia, Pennsylvania, USA; 5th International Conference on Medicinal Chemistry & Computer Aided Drug Designing December 01-03, 2016, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
Track 10: Future Hereditary and Rare Diseases e Prospects of Rare Diseases
Work over the past 25 years has resulted in the identification of genes responsible for ~50% of the estimated 7,000 rare monogenic diseases and it is predicted that most of the remaining disease-causing genes will be identified by the year 2020 and probably sooner. This marked acceleration is the result of dramatic improvements in DNA-sequencing technologies and the associated analyses. We examine the rapid maturation of rare-disease genetic analysis and successful strategies for gene identification. We highlight the impact of discovering rare-disease-causing genes, from clinical diagnostics to insights gained into biological mechanisms and common diseases. Last, we explore the increasing therapeutic opportunities and challenges that the resulting expansion of the 'atlas' of human genetic pathology will bring.
Related Conferences:
3rd Euro-Global Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 3rd Euro-Global Emerging Infectious Diseases Conferences September 5-6, 2016 Frankfurt, Germany, 2nd International Conference on Retroviruses & Novel Drugs June 30-July 01, 2016, South Africa; 5th Global Pharmacovigilance Summit April 28-29, 2016, UAE; 2nd Annual Congress on Pharma Middle East October 10-12, 2016; International Conference on Medical Ethics & Legal Medicine June 09-11, 2016 , UK; 2ndInternational Conference on Therapeutic Drug Monitoring and Toxicogenomics June 09-10, 2016, USA; 4th International Conference on HIV/AIDS, STDS and STIS October 03-05, 2016, USA; Infectious Diseases Conferences Europe, Frankfurt Germany, September 5-7 2016; 5th International Conference on Medicinal Chemistry & Computer Aided drug Designing December 01-03, 2016, Phoenix, Arizona, USA; 2nd International Conference and Exhibition on Pharmacology and Ethnopharmacology May 02-04, 2016, USA; 6th annual Outsourcing in Clinical Trials East Coast conference May 25-26 2016, USA; Outsourcing in Clinical Trials Europe 2016 May 17-18 2016, France; 5th Annual Clinical Development and Trials Asia Congress January 2016, china; ECRD European Conference for Rare Diseases and Orphan Products, May 26 - 28 2016 ; BPSU - Rare Disease Conference 23 February 2016; International Conferences on Rare Diseases and Orphan Drugs Cape, 19-22 October 2016,South Africa; Orphan Drugs Summit, 21st - 23rd September 2016, The Netherlands.
OMICS International Conferences invites all the participants from all over the world to attend ‘Annual Congress on Rare Diseases Orphan Drugs’ during Oct 26-27, 2016 in Chicago, USA which includes prompt keynote presentations, Oral talks, Poster presentations and Exhibitions.
Rare Diseases 2016 is a global platform to discuss and learn about Rare Diseases, rare cancer, Morgellons, Renal cell carcinoma, malignant glioma, Turner syndrome, Multiple myeloma, Hepatocellular carcinoma, Diabetes in the young, Systemic Amyloidosis, Autoimmune diseases, Disorders of genetic origin, idiopathic disorders, rare genetic diseases, Orphan Drugs, Alzheimer’s Disease and many more. OMICS International organizes a conference series of 1000+ Global Events inclusive of 300+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in USA, Europe & Asia with support from 1000 more scientific societies and publishes 700+ Open access journals which contains over 30000 eminent personalities, reputed scientists as editorial board members.
Why to attend?
In today's economic climate your business decisions are as crucial as ever. International conference on Rare Diseases and Orphan Drugs allow you to maximize your time and marketing dollars while receiving immediate feedback on your new products and services. International conference on Rare Diseases and Orphan Drugs is organizing an outstanding Scientific Exhibition/Program and anticipates the world's leading specialists involved Rare Diseases and Orphan Drugs. Your organization will benefit with excellent exposure to the leaders in Rare Diseases and Orphan Drugs. Rare Diseases-2016 is an exciting opportunity to showcase the new technology, the new products of your company, and/or the service your industry may offer to a broad international audience.
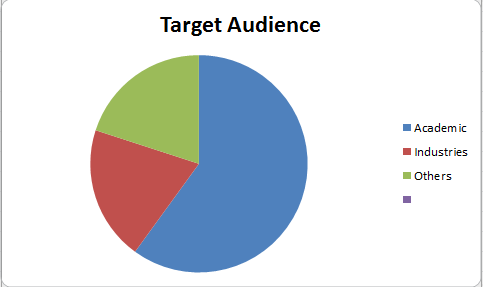
Target Audience
- Super specialists
- Specialists
- Researchers
- Students
- Industrial delegates
- Academia and Research
- Biomedical companies
- Healthcare Professionals
Summary
Rare Disease is a branch of medicine. Which are an important public health issue and challenge for the medical community. They are called “Health Orphan”. The term “Orphan Drug” refers to a drug or biologics such as vaccine or blood product that treats a rare disease or conditions. Rare Disease welcomes all the specialists and super specialists of medical field to discuss on rare diseases, diagnose and determine the severity of or treat a variety of diseases, including many types of rare cancers, Morgellons, Renal cell carcinoma, malignant glioma, Multiple myeloma, Hepatocellular carcinoma and other abnormalities within the body. This conference will witness the fusion of advanced technology in the field of Rare Diseases.
The organizing committee is gearing up for an exciting and informative conference program including plenary lectures, symposia, workshops on a variety of topics, poster presentations and various programs for participants from all over the world. We invite you to join us at the Rare Diseases-2016, where you will be sure to have a meaningful experience with scholars from around the world. All members of the Rare Diseases-2016 organizing committee look forward to meet you in Chicago, USA.
For more details please visit: http://rarediseases.conferenceseries.com/
Importance and Scope
Rare Diseases-2016 will be the best platform for all the specialists and super specialists, renowned Scientists, research scholars, students who are working in this field across the globe under a single roof to exchange their knowledge related to Rare Diseases and Orphan Drugs. This international event is an effort to find an alternative for invasive imaging technique against diseases like rare cancer, Morgellons, Renal cell carcinoma, malignant glioma, Multiple myeloma, Hepatocellular carcinoma etc.
OMICS Group welcomes all the specialists and super specialists, research scholars, industrial professionals and student delegates from biomedical and healthcare sectors to be a part of the esteemed Rare Diseases-2016. It also catalyses for information exchange and networking between researchers and business entrepreneurs of diverse backgrounds for the advancement of Technology and Research in the field of Rare Diseases. As this will be the best amalgamation of academia and research involving every aspect of empirical and conceptual thinking in exploring new dimensions in this field. It is open to all types of research methodologies both from academia and industry.
Why Chicago?
Rare Diseases-2016 is going to held in Chicago, a city in the U.S. state of Illinois, is the third most populous city in the United States and the most populous city in the American Midwest, with approximately 2.7 million residents. Its metropolitan area (also called "Chicago land"), which extends into Indiana and Wisconsin, is the third-largest in the United States, after those of New York City and Los Angeles, with an estimated 9.8 million people. Chicago is the county seat of Cook County, though a small portion of the city limits also extends into Du Page County.
Chicago was incorporated as a city in 1837, near a portage between the Great Lakes and the Mississippi River watershed. Today, Chicago is listed as an alpha+ global city by the Globalization and World Cities Research Network, and ranks seventh in the world in the 2012 Global Cities Index. The city is an international hub for finance, commerce, industry, telecommunications, and transportation, with O'Hare International Airport being the second-busiest airport in the world in terms of traffic movements.
In 2012, Chicago hosted 46.2 million international and domestic visitors. Among metropolitan areas, Chicago has the fourth-largest gross domestic product (GDP) in the world, just behind Tokyo, New York City, and Los Angeles, and ranking ahead of London and Paris. Chicago is one of the most important Worldwide Centers of Commerce and trade.
Chicago's notability has found expression in numerous forms of popular culture, including novels, plays, films, and songs. The city has many nicknames, which reflect the impressions and opinions about historical and contemporary Chicago. The best-known include "Windy City" and "Second City. Rare Diseases-2016 Conference at Chicago will certainly give a wonderful experience to attendees to explore the beautiful city with gaining knowledge.
Conference Highlights
- Different types of Rare Diseases
- Clinical Research and Public Awareness
- Mystery Diagnosis of Rare Diseases
- Challenges in Rare Diseases Treatment
- Orphan Drugs, Development trends and strategies
- Orphan Drugs and Ethical Issues
- Helping patients afford the high cost of orphan drugs-comparison with others
- Future Hereditary and Rare Diseases e Prospects of Rare Diseases
Why to attend?
In today's economic climate your business decisions are as crucial as ever. International conference on Rare Diseases and Orphan Drugs allow you to maximize your time and marketing dollars while receiving immediate feedback on your new products and services. International conference on Rare Diseases and Orphan Drugs is organizing an outstanding Scientific Exhibition/Program and anticipates the world's leading specialists involved Rare Diseases and Orphan Drugs. Your organization will benefit with excellent exposure to the leaders in Rare Diseases and Orphan Drugs. Rare Diseases-2016 is an exciting opportunity to showcase the new technology, the new products of your company, and/or the service your industry may offer to a broad international audience.
A Unique Opportunity for Advertisers and Sponsors at this International event http://rarediseases.conferenceseries.com/sponsors.php
Target Audience
Specialists and super specialists, researchers, students, industrial delegates from Academia and Research along with the industrial professionals from biomedical companies and healthcare sectors.
Hospitals Associated with Rare Diseases Research USA & Worldwide
- The Manton Center for Orphan Disease Research (Boston, USA)
- US hospital for rare disease research (USA)
- NORD (National Organization for Rare Disorders) (USA)
- Chicago Rare Disease Foundation (Chicago, USA)
- National Institute of Health (NIH) funds research consortia to study more than 200 rare diseases (USA)
- Children’s hospital of Pittsburgh (Center for Rare Disease Therapy) (USA)
- Boston Children’s Hospital (The Manton Center for Orphan Disease Research) (Boston, USA)
- Birmingham children’s Hospital (NHS Foundation Trust) (UK)
- The Children’s Hospital of Philadelphia (USA)
- Hospitals Associated with Rare Diseases Research Chicago
- Comer Children’s Hospital – University of Chicago
- Ann & Robert H. Lurie Children’s Hospital of Chicago
Major Associations Worldwide
- The Boler-Parseghian Center for Rare & Neglected Diseases
- Cystic Fibrosis Foundation
- European Union Committee of Experts on Rare Diseases
- Multiple Myeloma Research Foundation
- U.S. Food and Drug Administration
- RARE Foundation Alliance
- Birmingham children’s Hospital (NHS Foundation Trust)
- Japan Patient Association
- EURORDIS Rare Diseases Europe
- Royal Society of Medicine
- EveryLife Foundation for Rare Diseases
- Global Genes Allies in Rare Diseases
- Rare Diseases South Africa
- Short Bowel Syndrome Foundation
- Rare Diseases or Syndromes and Clinical Societies
- Rare Diseases Patient Association Funding
- Rare Disorders Society Singapore
- Guardian Hands Foundation
- IRDR Intractable & Rare Diseases Research (Europe)
Major Associations Chicago
- National Organization for Rare Diseases (NORD)
- Chicago Rare Disease Foundation
- The Boler-Parseghian Center for Rare & Neglected Diseases
- Alzheimer's Disease Organizations
- National Institute of Health
- Major Associations Worldwide
- Canadian Organization for Rare Diseases (CORD)
- National Alliances for Rare Diseases
- Organization for Rare Diseases India (ORDI)
Industries Associated with Rare Diseases Research Chicago
- AbbVie North Chicago
- Genus Oncology Vernon Hills
- Elorac Vernon Hills
- Baxter International Deerfield
- ViroPharma
- Dohmen Life Science Services Acquires Chicago Company
- Astellas Pharma US Northbrook
- Lundbeck Deerfield
- Marathon Pharmaceuticals
- Industries Associated with Rare Diseases Research Worldwide
- Novartis (Switzerland)
- Pfizer (USA)
- Roche (Switzerland)
- Onyx Pharmaceuticals
- Amicus Therapeutics
- Sarepta Therapeutics
- Prosensa
- Sanofi (France)
- Merck & Co. (USA)
- Swedish Orphan Biovitrum AB
- Genzyme
- AstraZeneca (UK)
- GlaxoSmithKline
- Vertex Pharmaceuticals
- NPS Pharmaceuticals
- Shire
- Johnson & Johnson (USA)
- ViroPharma
- Sigma-Tau Pharmaceuticals
- ViroPharma
- European Medicines Agency
- Takeda (Japan)
- Aegerion Pharmaceuticals
- Novo Nordisk (Denmark)
- Cancer Prevention Pharmaceuticals
- Roche
- PharmaMar USA
- Raptor Therapeutics
- Daiichi Sankyo (Japan)
- Araim Pharmaceuticals
- Octapharma USA
- Gilead sciences (USA)
- Millennium Pharmaceuticals
- Actavis (USA)
- Amgen (USA)
- Glaxosmithkline (UK)
- Bayer HealthCare (Germany)
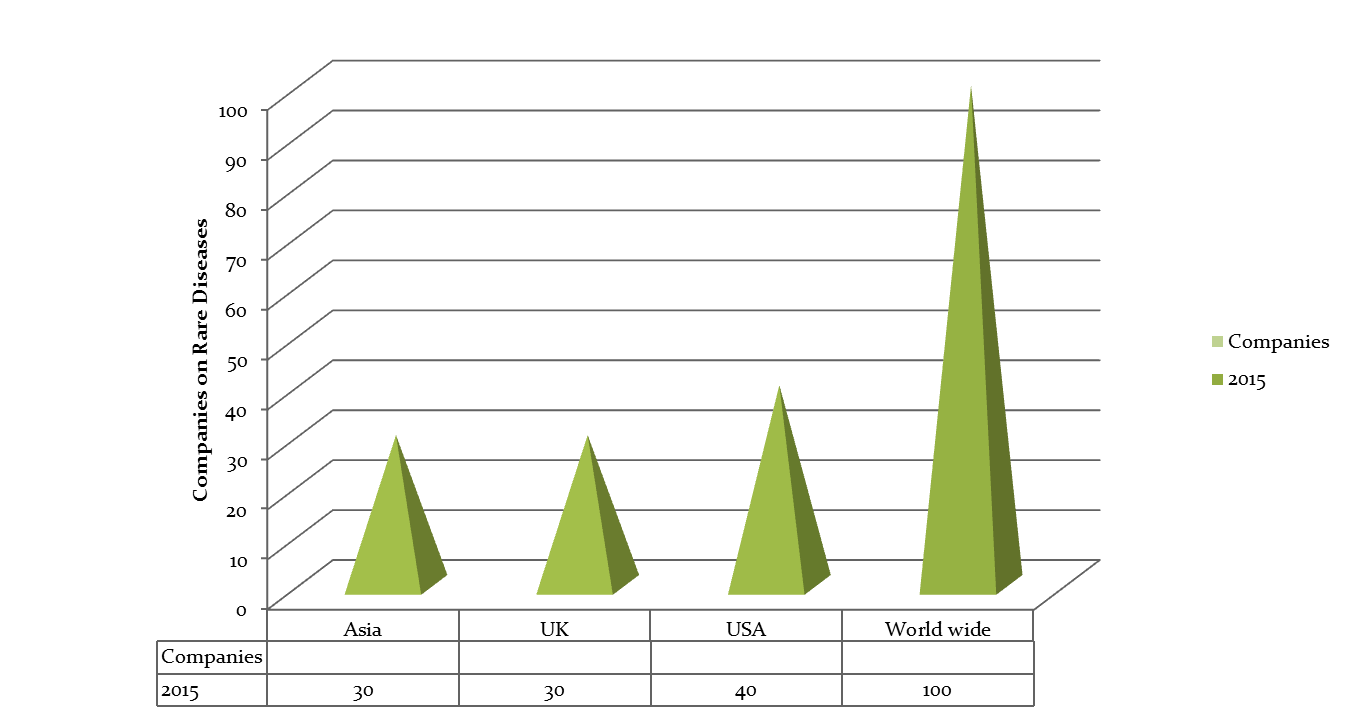
Top Universities in Chicago & USA
- University of California - San Francisco
- The University of Chicago Medicine
- The University of Chicago Celiac Disease Center
- University of Chicago (Neurofibromatosis Clinic)
- University Of Chicago Medical Center
- The University of Chicago Genetic Services
- University of Pennsylvania
- Northwestern University Feinberg School of Medicine
- Johns Hopkins University
- University of Pennsylvania
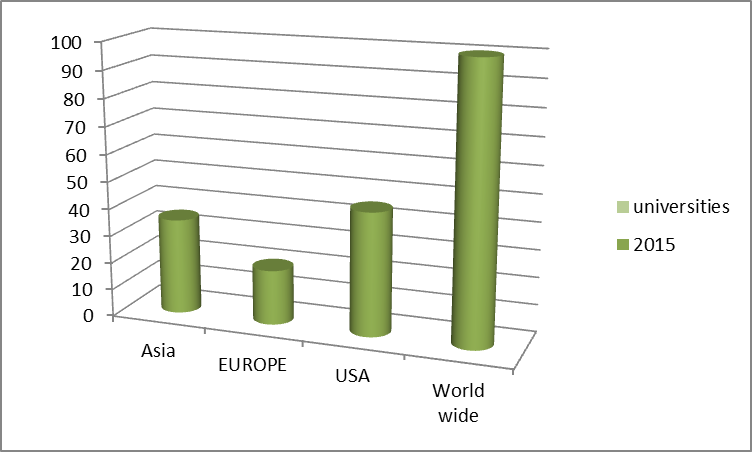
Top Universities in World Wide
- Harvard University
- Stanford University
- Washington University in St
- Yale University
- Columbia University
- University of Washington
- Duke University
- Top Universities Worldwide
- University of Oxford
- University of Cambridge
- Rare Genomics Institute
- GMEC, The Global Medical Excellence Cluster
- University of Zurich
- Cambridge University
- Yale University
- Emory University
- Karolinska University
- John Hopkins University
- Newcastle University
- University of Pittsburgh Study
- University of Valencia
- Osaka University
- McMaster University
- University of Celiac Disease Center
- Cincinnati Children's Hospital Medical Center
- Center for Rare Neurological Diseases (Northwestern University Feinberg School of Medicine)
Glance at Market analysis report
Summary GBI Research, the leading business intelligence provider, has released its latest research “Orphan Disease Therapeutics Market to 2018 – Improved Understanding of Rare Diseases’ Heterogeneity and Novel New Clinical Trial Designs to Foster Innovation”, which provides insights into the orphan disease therapeutics market until 2018. It includes the geographical distribution of Fabry, Pompe, Mucopolysaccharidosis VI, Idiopathic Thrombocytopenic Purpura, Huntington’s disease and Ovarian cancer markets across the US, the top five countries of Europe and in Japan. The report provides competitive benchmarking for the leading companies and also analyses the mergers, acquisitions and licensing agreements that shape the global markets. GBI Research’s analysis shows that the overall global orphan disease therapeutics market is expected to grow at a significant compound annual growth rate (CAGR) of 13.1% from $2.3 billion 2010 to $6 billion in 2018 in the US, the top five countries of Europe and Japan. Increasing awareness of the disease and drugs among patients and physicians, patent protection and exclusivity of Nplate and Promacta for ITP market, anticipated launch of new molecules such as ACR-16, AMR-101 and HD-02 for the treatment of HD and the approval of Avastin for the treatment of advanced ovarian cancer in Europe will drive the global orphan disease therapeutics market in the forecast period.
The 2013 Orphan Drug Report from Evaluate, which launched today at the 2013 BIO international Convention, sheds light on the market dynamics of orphan drugs — pharmaceutical products aimed at rare diseases or disorders — projecting that sales will experience a compound annual growth rate of 7.4 percent between 2012 and 2018, nearly double that of the prescription drug market, excluding generics. The report based on EvaluatePharma® data found that the worldwide orphan drug market is set to reach $127 billion by 2018, doubling that of the overall prescription drug market.
Market Growth of Rare Diseases Research
GBI Research’s analysis shows that the overall global orphan disease therapeutics market is expected to grow at a significant compound annual growth rate (CAGR) of 13.1% from $2.3 billion 2010 to $6 billion in 2018 in the US, the top five countries of Europe and Japan.
The 2013 Orphan Drug Report from Evaluate, which launched today at the 2013 BIO international Convention, sheds light on the market dynamics of orphan drugs — pharmaceutical products aimed at rare diseases or disorders — projecting that sales will experience a compound annual growth rate of 7.4 percent between 2012 and 2018, nearly double that of the prescription drug market, excluding generics. The report based on Evaluate Pharma data found that the worldwide orphan drug market is set to reach $127 billion by 2018, doubling that of the overall prescription drug market
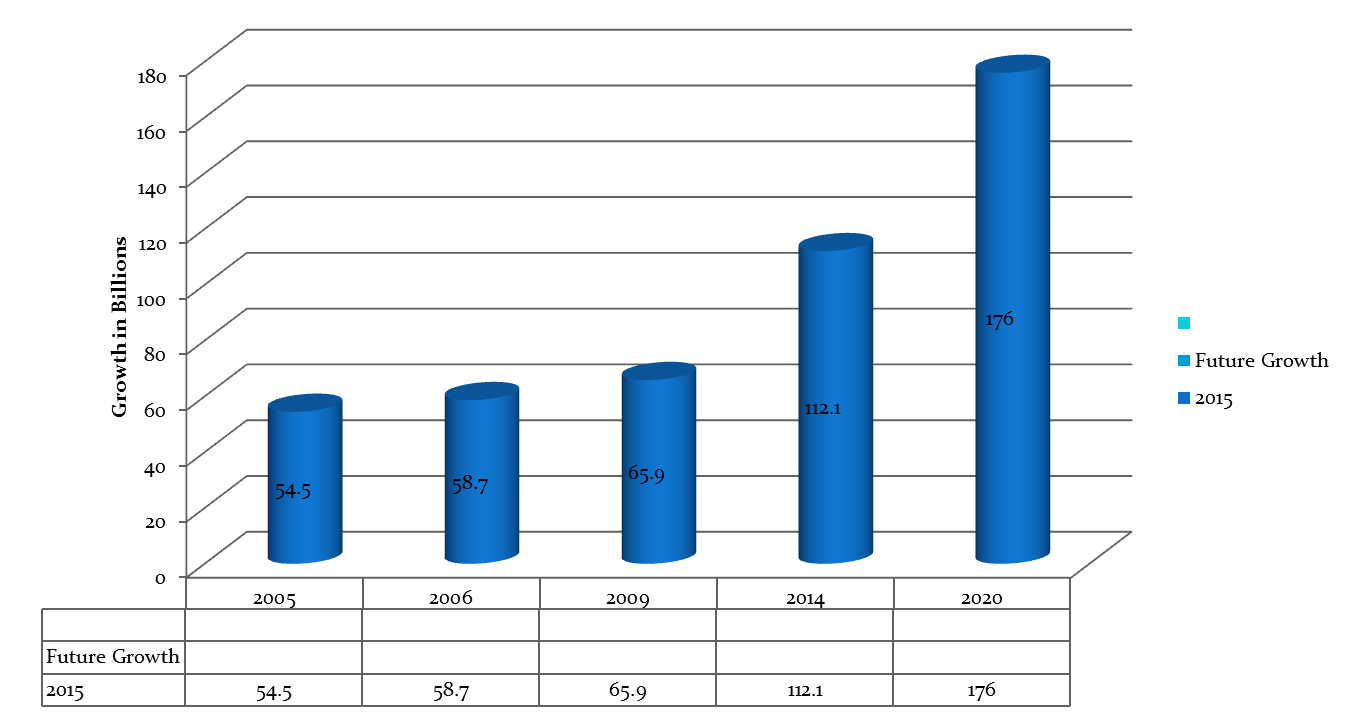
The global orphan drugs market reached $84.9 billion in 2009 growing from $58.7 billion in 2006 from $54.5 billion in 2005.The market is expected to grow at a compound annual growth rate (CAGR) of nearly 6% to reach $112.1 billion by 2014.The U.S. accounted for 51% of the market in 2009 and is expected to grow at a CAGR of 8.9% to reach $65.9 billion by 2014.By 2020, orphan drugs will own 19% of the total share of prescription drug sale excluding generics, reaching a whopping $176 billion in annual sales, according to Andreas Hadjivasiliou, an analyst with Evaluatepharma.
Fund Allotment to Rare diseases Research
Since 1989, NORD’s research grants have resulted in at least two FDA-approved products and the publication of numerous significant journal articles. Our more than 100 disease-specific research funds are supported primarily by patients and patient organizations. Industry support is welcome and would demonstrate commitment to the study of diseases for which there are few-if any- other sources of funding.
NORD's Research Program provides seed money grants to academic scientists for clinical studies related to the development of diagnostics or treatments of rare diseases. Requests for proposals are posted once a year in the late winter or early spring. NORD's Research Program also includes the NORD/Roscoe Brady Lysosomal Storage Diseases Fellowships.
Physician scientists at 22 consortia will collaborate with representatives of 98 patient advocacy groups to advance clinical research and investigate new treatments for patients with rare diseases. The collaborations are made possible through awards by the National Institutes of Health — totalling about $29 million in fiscal year 2014 funding — to expand the Rare Diseases Clinical Research Network (RDCRN), which is led by NIH’s National Center for Advancing Translational Sciences (NCATS).
References
http://www.raredisorders.ca/index.html
http://www.newshd.net/senza-categoria/1115/orphan-disease-therapeutics-market-to-2018-improved-understanding-of-rare-diseases-heterogeneity-and-novel-new-clinical-trial-designs-to-foster-innovation/
http://www.biotech-now.org/health/2013/04/worldwide-orphan-drug-market-to-grow-to-127-billion-by-2018
http://www.topuniversities.com/university-rankings/university-subject-rankings/2014/medicine#sorting=rank+region=+country=+faculty=+stars=false+search=
http://www.huffingtonpost.com/2014/03/11/best-medical-schools-2015_n_4935490.html?ir=India&adsSiteOverride=in
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2996062/
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2996062/
http://www.mmm-online.com/features/therapeutic-focus-rare-diseases/article/394333/
Conference Highlights
- Different types of Rare Diseases
- Clinical Research and Public Awareness
- Mystery Diagnosis of Rare Diseases
- Challenges in Rare Diseases Treatment
- Orphan Drugs, Development trends and strategies
- Orphan Drugs and Ethical Issues
- Rare Diseases in Aging
- Future Hereditary and Rare Diseases e Prospects of Rare Diseases
- Rare Diseases in Cancer
- Rare Infectious Diseases and Immune Deficiencies
- Entrepreneurs Investment Meet
- Clinical Immunology
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | Oct 26-27, 2016 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Trials
- Journal of Genetic Syndromes & Gene Therapy
- Journal of Neurological Disorders
Abstracts will be provided with Digital Object Identifier by